Why is the sky blue? It’s a common question asked by children, and the simple answer is that blue light is scattered by our atmosphere more than red light, hence the blue sky. That’s basically true, but then why don’t we see a violet sky?
The blue sky we observe depends upon two factors: how sunlight interacts with Earth’s atmosphere, and how our eyes perceive that light.
When light interacts with our atmosphere it can scatter, similar to the way one billiard ball can collide with another, making them go off in different directions. The main form of atmospheric scattering is known as Rayleigh scattering. If you imagine photons bouncing off molecules of air, that’s a rough approximation. But photons and air molecules aren’t billiard balls, so there are differences. One of these is that the amount of scattering depends upon the wavelength (or color) of the light. The shorter the wavelength, the more the light scatters. Since the rainbow of colors going from red to violet corresponds with wavelengths of light going from long to short, the shorter blue wavelengths are scattered more. So our sky appears blue because of all the scattered blue light. This is also the reason why sunsets can appear red. Blue light is scattered away, leaving a reddish looking sunset.
But if that’s the case, why isn’t the sky violet? Sure, blue light is scattered more than red or green, but violet light has an even shorter wavelength, so violet should be scattered more than blue. Shouldn’t the sky appear violet, or at least a violet-blue? It turns out our sky is violet, but it appears blue because of the way our eyes work.
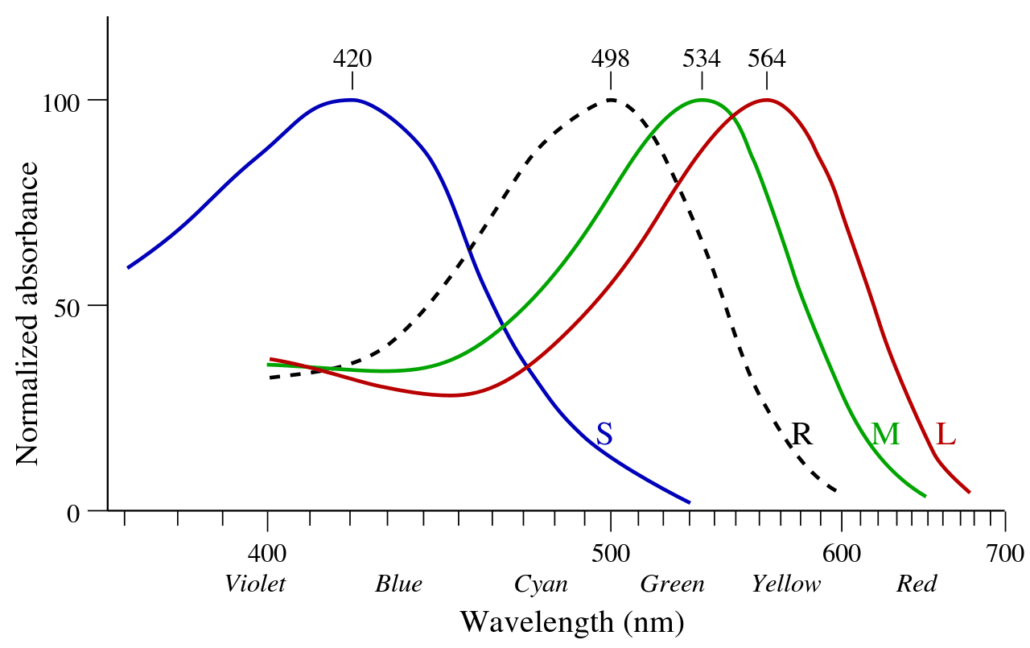
We don’t see individual wavelengths. Instead, the retinas of our eyes have three types of color sensitive cells known as cones. One type is most sensitive to red wavelengths, while the other two are most sensitive to green and blue wavelengths. When light we look at something, the strength of signal from each type of cone allows our brains to determine the colors we see. These colors roughly correspond to the actual wavelengths we see, but there are subtle differences. While each type of cone has its peak sensitivity at red, green, or blue, they also detect light of other colors. Light with “blue” wavelengths stimulate blue cones the most, but they also stimulate red and green just a little bit. If it really was blue light that was scattered most, then we’d see the sky as a slightly greenish blue.
We don’t see the greenish hue, however, because of the sky’s violet light. Violet is scattered most by Earth’s atmosphere, but the blue cones in our eyes aren’t as sensitive to it. While our red cones aren’t good at seeing blue or violet light, they are a bit more sensitive to violet than our green cones. If only violet wavelengths were scattered, then we would see violet light with a reddish tinge. But when you combine the blue and violet light of the sky, the greenish tinge of blue and reddish tinge of violet are about the same, and wash out. So what we see is a pale blue sky.
As far as wavelengths go, Earth’s sky really is a bluish violet. But because of our eyes we see it as pale blue.












Comments
I have heard/read somewhere that ozone, which is blue, contributes quite a lot to the blue color of the sky. I have no recollection of exactly how much though.
I don’t think so.
Ozone is a colourless gas but is produced by processes such as a corona discharge which has a characteristic blue colour.
The corona discharge ionizes gas producing electrons and positive ions.
These electrons can be recaptured by the positive ions emitting photons in the process resulting in the colour.
Ozone is definitely blue. It only looks colorless at low conc. It is said to be the cause of the blue tinge you see in liquid oxygen. There aŕe plenty of ozone pics available on the internet.
Even so, ozone content in our atmosphere is extremely low, and its contribution into overall color of the sky is negligible.
Your point is taken that ozone has a blue colour.
Given that ozone mostly exists in the stratosphere at average concentrations of around 0.6 ppm, the concentration is way too low to contribute to the sky colour.
The blue colour of liquid oxygen is due to absorption of photons at 650nm which excite the oxygen molecular bonds.
Light in the 650nnm range is deep red in colour, absorption of red light gives liquid oxygen its blue colour.